Identical Particles
Preamble
All intrinsic properties of the particles are the same. I.e. mass, charge, spin. E.g. 2e-, 2p, 2n.
Non-identical particles (distinguishable). E.g. pe-, u-e-.
Example 1
Collide two particles, 1 and 2. If we have a detector \(\mathcal{D}\) which detects the particles after the collision. Did it detect 1, 2, or both? Exchange-degeneracy.
Example 2
We have particle 1 at \(x=a\) and particle 2 at \(x=b\). If they are distinguishable then exchanging them gives a different setup: \(|a_{m_1}b_{m_2}\rangle \neq |a_{m_2}b_{m_1}\rangle\). If they are indistinguishable then exchanging them gives the same setup: \(|a_{m_1}b_{m_2}\rangle = |a_{m_2}b_{m_1}\rangle\).
Definition
The permutation operator \(\hat{\mathcal{P}}_{ij}\) is defined by \(\hat{\mathcal{P}}_{ij}\Psi(\vec{r}_1,\varepsilon_1;\vec{r}_2,\varepsilon_2;\cdots;\vec{r}_j,\varepsilon_j;\vec{r}_j,\varepsilon_j;\cdots;\vec{r}_N,\varepsilon_N) = \lambda\Psi(\vec{r}_1,\varepsilon_1;\vec{r}_2,\varepsilon_2;\cdots;\vec{r}_j,\varepsilon_j;\vec{r}_i,\varepsilon_i;\cdots;\vec{r}_N,\varepsilon_N)\) with \(\lambda \in \{-1, 1\}\). The \(\lambda\) can be found by applying the permutation operator twice.
For \(\lambda=1\), we have symmetric wavefunctions, bosons, integer spin, e.g. \(H\), \(D\) (Deuteron), \(\gamma, He, 2p+2n\). For \(\lambda=-1\), we have antisymmetric wavefunctions, fermions, half-integer spin, e.g. \(e^-,p,n,^{13}C\).
Can a fermion become a boson through an interaction, i.e. is there a \(V\) such that \(\langle \Psi_S|V|\Psi_A\rangle\neq 0\)? If this was the case then applying the parity operator twice on either side, \(\langle\Psi_S|\hat{\mathcal{P}}V\hat{\mathcal{P}}(-|\Psi_A\rangle)=\langle\Psi_S|V|\Psi_A\rangle\). Thus, the matrix element must be zero.
N-Particles
\(|\Psi_S\rangle = \frac{1}{\sqrt{N!}}\left(|\Psi_1\Psi_2\cdots\Psi_N\rangle + |\Psi_2\Psi_1\cdots\Psi_N\rangle + \cdots + |\Psi_1\Psi_2\cdots\Psi_N\Psi_{N-1}\rangle + \cdots\right)\). The \(N!\) comes from the fact that there are \(N!\) factorial ways to permute.
\(|\Psi_A\rangle\) is given by Slater’s determinant. I.e. for the 3x3 case: \(\frac{1}{\sqrt{N!}}\begin{vmatrix} \Psi_1(\vec{r}_1,\varepsilon_1) & \Psi_2(\vec{r}_1,\varepsilon_1) & \Psi_3(\vec{r}_1,\varepsilon_1) \\ \Psi_1(\vec{r}_2,\varepsilon_2) & \Psi_2(\vec{r}_2,\varepsilon_2) & \Psi_3(\vec{r}_2,\varepsilon_2) \\ \Psi_1(\vec{r}_3,\varepsilon_3) & \Psi_2(\vec{r}_3,\varepsilon_3) & \Psi_3(\vec{r}_3,\varepsilon_3) \end{vmatrix} = \frac{1}{\sqrt{N!}}\left[\Psi_1(\Psi_2\Psi_3-\Psi_3\Psi_2) - \Psi_2(\Psi_1\Psi_3-\Psi_3\Psi_1) + \Psi_3(\Psi_1\Psi_2-\Psi_2\Psi_1)\right]\).
Distinguishable: \(|\Psi\rangle = \prod\limits_n^N|\Psi_n\rangle\).
Permutations
Given \(N\) particles and \(N\) boxes then we have \(N!\) possible combinations to fill the boxes.
Given \(N\) particles and \(k\) boxes then we have \(\frac{N!}{(N-k)!} = k!{N\choose k}\) possible permutations to fill the boxes.
How many $k-$combinations can we get? With combinations the boxes are unlabled hence ab and ba are the same. Then we have \({N\choose k}\).
Note: \((x+y)^N = \sum_{k=0}^N {N\choose k}x^ky^{N-k}\).
Two Particles in a Box
\(E_{tot} = \frac{\hbar^2\pi^2}{mL^2}\). For each particle, \(\frac{\hbar^2\pi^2}{2mL^2}(n_1^2+n_2^2)\). Then, we have \(|11\rangle\) which is allowed for distinguishable and bosonic particles.
\(E_{tot} = \frac{5\hbar^2\pi^2}{2mL^2}\). For each particle, \(\frac{\hbar^2\pi^2}{2mL^2}(n_1^2+n_2^2)\). Then, we have \(|12\rangle,|21\rangle\) which is allowed for distinguishable. Boson: \(1/\sqrt{2}(|12\rangle+|21\rangle)\). Fermion: \(1/\sqrt{2}(|12\rangle-|21\rangle)\).
For more degrees of freedom, say spin, then a symmetric function would be both the coordinate and spin being symmetric or antisymmetric.
Z Electron Atom
We will not consider relativisitic corrections, and have spin-dependent terms and a stationary nucleus.
\(H = \sum_i^Z \frac{p_i^2}{2m} - \sum_i^Z \frac{Ze^2}{R_i} + \sum_{i
If we didn’t have the last term, \(H = \sum_i^Z H_i\).
Hence, \(\Psi = \Psi_1\cdots\Psi_Z\).
How many terms does the last term have?
\(\frac{Z(Z-1)}{2}={Z\choose 2}\) terms.
The second term is roughly, \(\frac{Ze^2}{R}Z\) and the third is roughly \(\frac{e^2}{R}\frac{Z(Z-1)}{2}\).
The second term is 4 times larger for helium, not a great perturbation.
As we increase \(Z\), we approach 2, which is definitely a great perturbation.
Central Field Approximation
\(H = \sum_i^Z \left(\frac{p_i^2}{2m} + V_c(\vec{R}_i)\right)+V\) where \(V=-\sum_i^Z \frac{Ze^2}{R_i} + \sum_{i
So, if we can construct \(V_C\) such that \(V\) is minimal, it becomes an excellent perturbation.
Then, with the first term we have \(\Psi=R_n^\ell(r)Y_\ell^m(\Theta,\phi)\).
Considering small \(R_i\), the nuclear interaction potential should be just \(-\frac{Ze^2}{R_i}\).
For large \(R_i\), we have a nucleus of \(+Ze\) and the sea of electrons give a \(-(Z-1)e\). Then we see a net interaction charge of \(-\frac{e^2}{R_i}\).
For hydrogenic atoms, \(E = \sum_i^Z E_i\) with \(E_i=E_n\).
For non-hydrogenic atoms, \(E_i=E_n^\ell\).
The set of individual states with same \(E_n^\ell\) are called shells and occupied shells are electronic configuration.
We have \(2(2\ell+1)\) degeneracy. Hence how many electrons can occupy each shell.
For $s$-electrons \(\ell=0\) hence we can only have 2 electrons.
For $p$-electrons \(\ell=1\) hence we can only have 6 electrons.
For $d$-electrons \(\ell=2\) hence we can only have 10 electrons.
Outer shell matters most for interactions.
The ground state of helium is \(1s^2\): \(2E_{1s}\) the excited state is \(1s^12s^1\): \(E_{1s}+E_{2s}\) the second excited state is \(1s^12p^1\): \(E_{1s}+E_{2p}\).
If \(n_1\neq n_2\) or \(\ell_1\neq \ell_2\) then we have \(2(2\ell_1+1)\cdot 2(2\ell_2+1) = 4(2\ell_1+1)(2\ell_2+1)\) degeneracy.
Then the degeneracy of \(1s^12s^1\) is \(4(2\cdot0+1)(2\cdot0+1) = 4\).
If \(n_1=n_2\) and \(\ell_1=\ell_2\) then we have \({2(2\ell+1)\choose2}=(2\ell+1)(4\ell+1)\) total degeneracy.
The Helium Atom
\(H_0\Psi = E\Psi\), \(H_0 = H_1 + H_2\), \(H = \frac{p_1^2}{2m} + \frac{p_2^2}{2m} + \frac{e^2}{|\vec{R}_1-R_2|} - \frac{2e^2}{R_1} - \frac{2e^2}{R_2} = H_0 + V_p\), \(E = E_1^{n_1\ell_1} + E_2^{n_2\ell_2}\). Then, \(H_0 = \frac{p_1^2}{2m} + \frac{p_2^2}{2m} + V_c(R_1) + V_c(R_2)\).
Degeneracy:
- \(n_1\neq n_2\vee\ell_1\neq\ell_2\): \(4(2\ell_1+1)(2\ell_2+1)\)
- \(n_1=n_2\wedge\ell_1=\ell_2\): \((2\ell+1)(4\ell+1)\)
If we had a \(1s^2\) state then we would have \(|\Psi\rangle = \frac{1}{\sqrt{2}}\Psi_{n_1\ell_1m_1}\left(|+-\rangle - |-+\rangle\right)|100;100\rangle = \Psi_{1s}(\vec{r}_1)\Psi_{1s}(\vec{r}_2)\frac{1}{\sqrt{2}}(|+-\rangle - |-+\rangle)\). Spatial antisymmetric and spin symmetric OR Spatial symmetric and spin antisymmetric. The spin antisymmetric is only possible for the same spatial quantum numbers.
\(V_p = -\frac{2e^2}{R_1} - \frac{2e^2}{R_2} + \frac{e^2}{|\vec{R}_1-\vec{R}_2|} - V_c(R_1) - V_c(R_2)\).
Consider the 1s + 2s configuration. For our 4-fold degenerate space, we must find the 4 wavefunctions. \(|^1S\rangle = |\Psi_{spatial,S}\rangle\otimes|\Psi_{spin,A}\rangle = \Psi_{S}\otimes|s=0,m_S=0\rangle = \Psi_{S}\otimes|0,0\rangle = \Psi_{S}\otimes\frac{1}{\sqrt{2}}(|+-\rangle - |-+\rangle)\). \(|^2S\rangle = |\Psi_{spatial,A}\rangle\otimes|\Psi_{spin,S}\rangle = \frac{1}{\sqrt{2}}(|100;200\rangle - |200;100\rangle)\otimes\begin{cases}|10\rangle\\|11\rangle\\|1-1\rangle\end{cases}\).
So, \(\langle\Psi|V|\Psi\rangle = \langle\Psi_{spatial}|V|\Psi_{spatial}\rangle\). Note that the spin is unperturbed by our potential. From the spins, we see that our perturbation is already diagonal. I will call our two spatial wavefunctions by the spectroscopic notation. \(\langle ^1S|V|^1S\rangle,\langle ^3S|V|^3S\rangle\).
Then, \(\langle V\rangle = \frac{1}{2}\left[\int d^3\vec{r}_1d^3\vec{r}_2|\Psi_{1s}(\vec{r}_1)|^2V|\Psi_{2s}(\vec{r}_2)|^2 + \int d^3\vec{r}_1d^3\vec{r}_2|\Psi_{1s}(\vec{r}_2)|^2V|\Psi_{2s}(\vec{r}_1)|^2 \pm \int d^3\vec{r}_1d^3\vec{r}_2\Psi_{1s}^*(\vec{r}_1)\Psi_{2s}^*(\vec{r}_2)V\Psi_{1s}(\vec{r}_2)\Psi_{2s}(\vec{r}_1) \pm \int d^3\vec{r}_1d^3\vec{r}_2\Psi_{1s}^*(\vec{r}_2)\Psi_{2s}^*(\vec{r}_1)V\Psi_{1s}(\vec{r}_1)\Psi_{2s}(\vec{r}_2)\right] = \int d^3\vec{r}_1d^3\vec{r}_2|\Psi_{1s}(\vec{r}_1)|^2V|\Psi_{2s}(\vec{r}_2)|^2 \pm \int d^3\vec{r}_1d^3\vec{r}_2\Psi_{1s}^*(\vec{r}_1)\Psi_{2s}^*(\vec{r}_2)V\Psi_{1s}(\vec{r}_2)\Psi_{2s}(\vec{r}_1)\). Note that \(V\) has five terms so we get a trivial 10 integrals to evaluate.
Consider the first integral.
\begin{align*} \int d^6\vec{r}|\Psi_{1s}(\vec{r}_1)|^2V|\Psi_{2s}(\vec{r}_2)|^2 &= -2e^2\left(\int d^3\vec{r}_1\frac{|\Psi_{1s}(\vec{r}_1)|^2}{r_1} + \int d^3\vec{r}_2\frac{|\Psi_{1s}(\vec{r}_2)|^2}{r_2}\right) \\ &+ e^2 \int d^6\vec{r} \frac{|\Psi_{1s}(\vec{r}_1)|^2|\Psi_{2s}(\vec{r}_2)|^2}{|\vec{r}_1-\vec{r}_2|} \\ &- \int d^3\vec{r}_1|\Psi_{1s}(\vec{r}_1)|^2V_c(r_1) - \int d^3\vec{r}_2|\Psi_{2s}(\vec{r}_2)|^2V_c(r_2) \\ &= -2e^2I' + e^2K' - I_1 - I_2 = I + K - I_1 - I_2. \end{align*}The cross terms,
\begin{align*} \int d^3\vec{r}_1d^3\vec{r}_2\Psi_{1s}^*(\vec{r}_1)\Psi_{2s}^*(\vec{r}_2)V\Psi_{1s}(\vec{r}_2)\Psi_{2s}(\vec{r}_1) &= -2e^2\left(\int d^3\vec{r}_1\frac{\Psi_{1s}(\vec{r}_1)\Psi_{2s}(\vec{r}_1)}{r_1}\int d^3\vec{r}_2\Psi_{2s}(\vec{r}_2)\Psi_{1s}(\vec{r}_2) + \int d^3\vec{r}_2\frac{\Psi_{1s}(\vec{r}_2)\Psi_{2s}(\vec{r}_2)}{r_2}\int d^3\vec{r}_1\Psi_{2s}(\vec{r}_1)\Psi_{1s}(\vec{r}_1)\right) (=0) \\ &+ e^2 \int d^6\vec{r} \frac{\Psi_{1s}(\vec{r}_1)\Psi_{2s}(\vec{r}_2)\Psi_{1s}(\vec{r}_2)\Psi_{2s}(\vec{r}_1)}{|\vec{r}_1-\vec{r}_2|} \\ &- \int d^3\vec{r}_1\Psi_{1s}(\vec{r}_1)\Psi_{2s}(\vec{r}_1)V_c(r_1)\int d^3\vec{r}_2\Psi_{1s}(\vec{r}_2)\Psi_{2s}(\vec{r}_2) - \int d^3\vec{r}_1\Psi_{1s}(\vec{r}_1)\Psi_{2s}(\vec{r}_1)V_c(r_1)\int d^3\vec{r}_1\Psi_{1s}(\vec{r}_1)\Psi_{2s}(\vec{r}_1) \\ &= -2e^20 + e^2J' - 0 - 0 = J. \end{align*}Note that \(I_1, I_2\) are average energy for the central field for each electron. \(J\) is the exchange integral and is purely Quantum Mechanical.
Therefore, our full energies are \(E_+ = I-I_1-I_2+K+J\) and \(E_- = I-I_1-I_2+K-J\). Thus, we get a 4-fold degeneracy split into 1-fold and 3-fold degeneracy split by the exchange interaction.
So, if \(V_c(r_i) = -\frac{2e^2}{r_i}\) then \(V = \frac{e^2}{|\vec{r}_1-\vec{r}_2}\) and \(I-I_1-I_2 = 0\), Energies become \(K\pm J\). But note that this only contributes to an energy shift, not the split.
The singlet state has the higher energy, \(E_+\) since it is the symmetric spin wavefunction. The triplet state has the higher energy, \(E_-\) since it is the antisymmetric spin wavefunction.
In the context of helium, the singlet state is called parahelium and the triplet is orthohelium.
Spectroscopic Notation
Recall: \(^{2s+1}L\) where s is total spin and L is total orbital. So, \(1s^2\) gives \(^{1}S\) which as degeneracy \((2L+1)(2s+1) = 1\).
Ansatz: \(2p+2p \Rightarrow ^1S,^1D,^3P\Rightarrow 3 + 3 + 9 = 15\)
For \(1s+2s\) we have a non-degenerate \(^1S\) and 3-fold \(^3S\).
\(1s^2\Rightarrow\ell_1=0,\ell_2=0,s_1=1/2\Rightarrow L=0,S=0\Rightarrow ^1S\).
\(^{2s+1}L_J\) is also Spectroscopic, hence \(1s^2\Rightarrow ^1S_0\).
NOTE Full shells are non-degenerate since all possible states are already taken \(\Rightarrow ^1L\). Also, note that holes = electrons. Hence \(2p^5=2p\).
Hydrogen Molecule
Consider 2 hydrogen nuclei (a,b) with an electron each (1,2).
So, \(\vec{r}_{a1}\) is the vector between nucleus a and electron 1. Our total set of vectors are: \(\vec{r}_{a1},\vec{r}_{a2},\vec{r}_{ab},\vec{r}_{b1},\vec{r}_{b2},\vec{r}_{12}\).
The Hamiltonian is then, \(-\frac{\hbar^2}{2m_e}(\nabla_1^2 + \nabla_2^2) + e^2\frac{1}{r_{12}} - e^2\left(\frac{1}{\vec{r}_{a1}} + \frac{1}{\vec{r}_{a2}} + \frac{1}{\vec{r}_{b1}} + \frac{1}{\vec{r}_{b2}}\right)\) Let \(r_{ab}\) be a parameter of the system.
If \(r_{ab}\to\infty\) then \(H_{a1}=-\frac{\hbar^2}{2m_e}\nabla_1^2 - \frac{e^2}{r_{a1}},H_{b2} = -\frac{\hbar^2}{2m_e}\nabla_2^2 - \frac{e^2}{r_{b2}}\). Thus, \(H_0 = H_{a1} + H_{b2}\). The perturbation is then, \(V = \frac{e^2}{r_{12}} - e^2\left(\frac{1}{r_{a2}} + \frac{1}{r_{b1}}\right)\).
So, \(H_0\Psi_1 = E_0\Psi_1\), \(\Psi_1 = \Psi_a(\vec{r}_{a1})\Psi_b(\vec{r}_{b2})\). Then, \(H_0\Psi_1 = E_0\Psi_1 = 2E_I\Psi_1\).
The exchanged hamiltonian is then \(H_0' = H_{a2} + H_{b1}\). \(H_{a2} = -\frac{\hbar^2}{2m}\nabla_{2}^2 -\frac{e^2}{r_{a2}}, H_{b1} = \cdots\). Then, \(\Psi_2 = \Psi_a(\vec{r}_{a2})\Psi_b(\vec{r}_{b1})\) with the same energy, \(H_0'\Psi_2 = 2E_I\Psi_2\).
Then the full Hamiltonian is \(H = H_0 + V_{a1,b2} = H_0' + V_{a2,b1}\).
Our full wavefunction is then \(c_1\Psi_1 + c_2\Psi_2 + \phi\). Note that we will show \(|c_2| = |c_1|\).
\(H_1\Psi = E\Psi = (2E_I+\Delta E)\Psi\).
\begin{align} c_1H\Psi_1 + C_2H\Psi_2 + H\phi &= (2E_1+\Delta E)(c_1\Psi_1 + c_2\Psi_2+\phi) \\ c_1V_{a1,b2}\Psi_1 + c_2V_{a2,b1}\Psi_2 + H\phi &= 2E_1\phi + (\Delta E)(c_1\Psi_1 + c_2\Psi_2 + \phi) \nonumber \\ c_1V_{a1,b2}\Psi_1 &= \Delta Ec_1\Psi_1 \\ c_2V_{a2,b1}\Psi_2 &= \Delta Ec_2\Psi_2 \\ H\phi &= (2E_1 + \Delta E)\phi \end{align}Reducing by neglecting second-order perturbation,
\begin{align} c_1V_{a1,b2}\Psi_1 &= \Delta Ec_1\Psi_1 \\ c_2V_{a2,b1}\Psi_2 &= \Delta Ec_2\Psi_2 \\ H_0\phi = H_0'\phi &= 2E_1 \phi \end{align}Then,
\begin{align} (H_0-2E_I)\phi &= (\Delta E-V_{a1,b2})c_1\Psi_1 + (\Delta E-V_{a2,b1})c_2\Psi_2. \end{align}Note the RHS is the inhomogeneity. If RHS is zero then, \(H_0\phi - 2E_1\phi\Rightarrow \phi = \Psi_1\) if \(H_0\) and \(\phi=\Psi_2\) if \(H_0'\).
For an inhomogenous equation, there is a solution only if the RHS is orthogonal to the solution of the homogenous equation. Then,
\begin{align} \int [(\Delta E-V_{a1,b2})c_1\Psi_1 + (\Delta E-V_{a2,b1})c_2\Psi_2]^*\Psi_1 d^3\vec{r}_1d^3\vec{r}_2 &= 0 \\ \int [(\Delta E-V_{a1,b2})c_1\Psi_1 + (\Delta E-V_{a2,b1})c_2\Psi_2]^*\Psi_2 d^3\vec{r}_1d^3\vec{r}_2 &= 0 \end{align}Let,
\begin{align} K &= \int V_{a1,b2}|\Psi_1|^2d^3\vec{r}_1d^3\vec{r}_2 \\ K &= \int V_{a2,b1}|\Psi_2|^2d^3\vec{r}_1d^3\vec{r}_2 \\ \Delta E &= \int \Delta E|\Psi_{1,2}|^2 d^3\vec{r}_1d^3\vec{r}_2 \\ 0>J &= \int V_{a2,b1}\Psi_2^*\Psi_1d^3\vec{r}_1d^3\vec{r}_2 \\ 0>J &= \int V_{a1,b2}\Psi_1^*\Psi_2d^3\vec{r}_1d^3\vec{r}_2 \end{align}Gives us,
\begin{align} \Delta E c_1 - c_1K + \Delta E c_2\int \Psi_2^*\Psi_1 d^3\vec{r}_1d^3\vec{r}_2 - c_2J &= 0 \\ \Delta E c_1 - c_1K + \Delta E c_2|s|^2 - c_2J &= 0 \\ \Delta E c_2 - c_2K + \Delta E c_1\int \Psi_1^*\Psi_2 d^3\vec{r}_1d^3\vec{r}_2 - c_1J &= 0 \Delta E c_2 - c_2K + \Delta E c_1|s|^2 - c_1J &= 0 \end{align}So,
\begin{align} \Psi_1^*\Psi_2 d^3\vec{r}_1d^3\vec{r}_2 &= \int \Psi_a^*(\vec{r}_{a1})\Psi_b(\vec{r}_{b1})d^3\vec{r}_1\int\Psi_b^*(\vec{r}_{b2})\Psi_a(\vec{r}_{a2})d^3\vec{r}_2 \\ &= SS^* \\ &= |S|^2. \end{align}Gives us,
\begin{align} [\Delta E - K]c_1 + [\Delta E |S|^2 - J]c_2 &= 0 \\ [\Delta E - K]c_2 + [\Delta E |S|^2 - J]c_1 &= 0. \begin{vmatrix} \Delta E-K & \Delta E|S|^2 - J \\ \Delta E|S|^2 - J & \Delta E - K \end{vmatrix} &= 0 \\ (\Delta E)_1 &= \frac{K+J}{1+|S|^2} \\ (\Delta E)_2 &= \frac{K-J}{1+|S|^2}. \end{align}The second is the symmetric solution \(c_1=c_2\).
\(\Psi_{S,Spatial} = \frac{1}{\sqrt{2(1 + |S|^2)}}(\Psi_1 + \Psi_2)\). Then, \(E_S = 2E_1 + \frac{e^2}{r_{ab}} + \frac{K-J}{1+|S|^2} = 2E_1 + \frac{e^2}{r_{ab}} + K + J - |S|^2\frac{K+J}{1+|S|^2}\), which also includes the proton-proton energy. The second form is written so that you can treat the last term as a small correction.
\(\Psi_{A,Spatial} = \frac{1}{\sqrt{2(1-|S|^2)}}(\Psi_1-\Psi_2)\). \(E_A = 2E_1 + \frac{e^2}{r_{ab}} + \frac{K+J}{1+|S|^2} = 2E_1 + \frac{e^2}{r_{ab}} + K - J - |S|^2\frac{K-J}{1-|S|^2}\). Thus, the exchange energy is the differentiating factor and since \(J<0\) the antisymmetric wavefunction is higher energy.
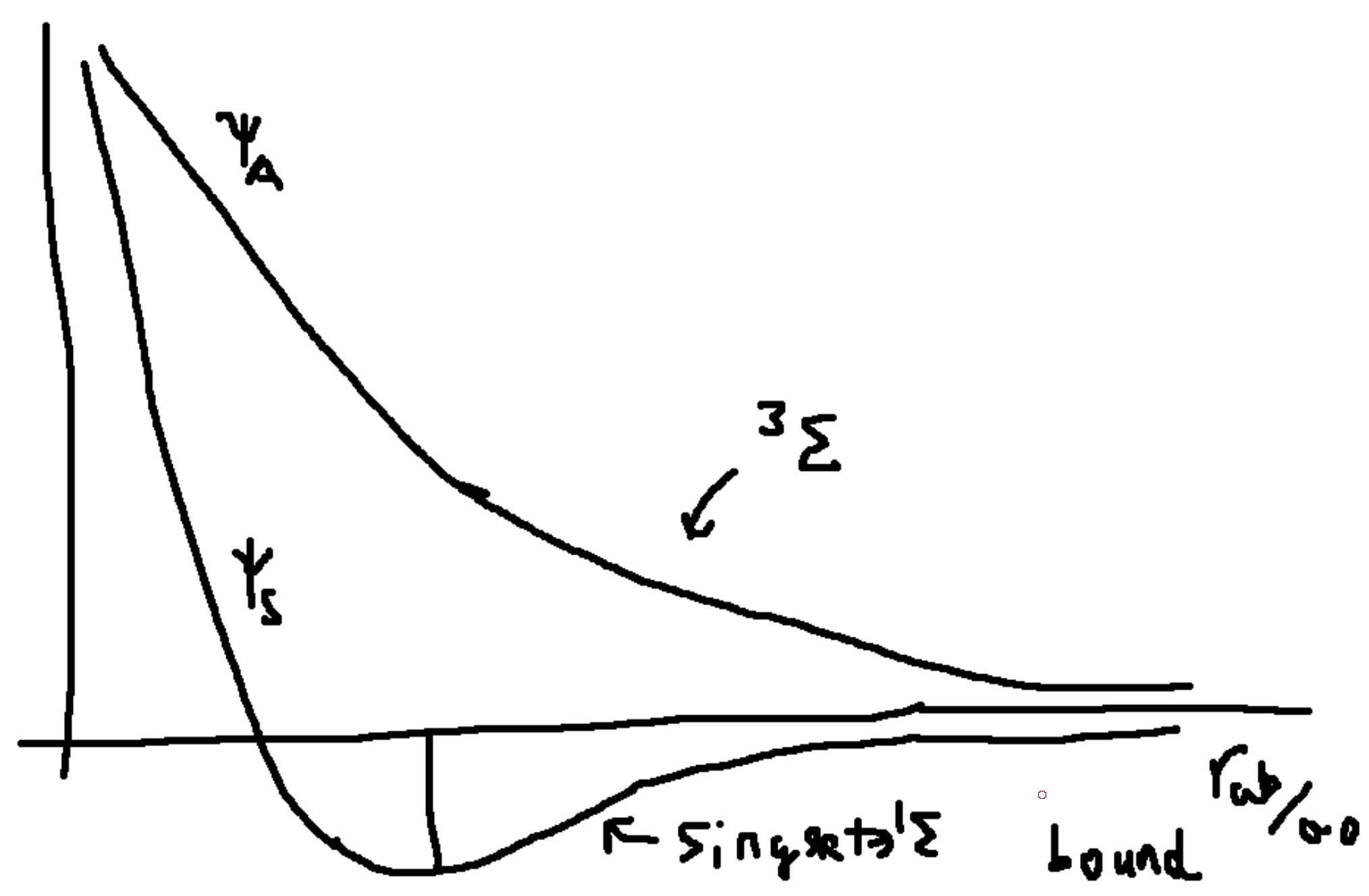
Bond length: \(r_{ab,0}\approx 1.4 a_0\), where the verticle reaches the x-axis. The bottom of the well is like a harmonic oscillator with \(\frac{\hbar\omega_0}{2}\) given by the curvature of the bottom of the well: \(\omega_0 = \sqrt{\frac{1}{m}\frac{d^3E_S}{dr_{ab}^2}}\). The height is the Diassociation energy \(D=4.38\) eV.
| Theory | Experiment | |
| \(r_{ab}\) | 0.735 A | 0.753 A |
| \(\omega_0\) | 4280 1/cm | 4390 1/cm |
| \(D\) | 4.37 eV | 4.38 eV |
Time Dependence
Consider, \(H_1\varphi_a(\vec{r}_1) = E_a\varphi_a(\vec{r}_1)\) and \(H_2\varphi_b(\vec{r}_2) = E_b\varphi_b(\vec{r}_2)\). \((H_1 + H_2 + H_{12})\Psi = E\Psi\) \(\Psi_{A,S} = \frac{1}{\sqrt{2}}\left(\varphi_a(\vec{r}_1)\varphi_b(\vec{r}_2)\mp\varphi_b(\vec{r}_1)\varphi_a(\vec{r}_2)\right)\). Then, \(E_{A,S} = E_A + E_B + K\mp J\). Consider, \(\Psi(0) = \varphi_a(\vec{r}_1)\varphi_b(\vec{r}_2) = \frac{1}{\sqrt{2}}\left(\Psi_A(0) + \Psi_S(0)\right)\). At what time is the occupation reversed, \(\Psi(t) = \varphi_a(\vec{r}_2)\varphi_b(\vec{r}_1)\)?
From the superposition, we can time-evolve the state and find when the occupation is reversed,
\begin{align} \Psi(t) &= \frac{1}{\sqrt{2}}\left(\Psi_S(0)\exp\left(-\frac{i}{\hbar}E_St\right) + \Psi_A(0)\exp\left(-\frac{i}{\hbar}E_At\right)\right) \nonumber \\ &= \frac{1}{\sqrt{2}}\left(-\frac{i}{\hbar}(E_a+E_b+K)t\right)\left(\Psi_S(0)\exp\left(-\frac{i}{\hbar}Jt\right)+\Psi_A(0)\exp\left(\frac{i}{\hbar}Jt\right)\right) \nonumber \\ &= \frac{1}{2}\left(-\frac{i}{\hbar}(E_a+E_b+K)t\right)\left(\left(\varphi_a(\vec{r}_1)\varphi_b(\vec{r}_2)+\varphi_a(\vec{r}_2)\varphi_b(\vec{r}_1)\right)\exp\left(-\frac{i}{\hbar}Jt\right)+\left(\varphi_a(\vec{r}_1)\varphi_b(\vec{r}_2)-\varphi_a(\vec{r}_2)\varphi_b(\vec{r}_1)\right)\exp\left(\frac{i}{\hbar}Jt\right)\right) \nonumber \\ &= \exp\left(-\frac{i}{\hbar}(E_a+E_b+K)t\right)\left(\cos\frac{Jt}{\hbar}\varphi_a(\vec{r}_1)\varphi_b(\vec{r}_2)-i\sin\frac{Jt}{\hbar}\varphi_b(\vec{r}_1)\varphi_a(\vec{r}_2)\right) \end{align}this is reversed when \(t=\frac{\pi\hbar}{2J}\).